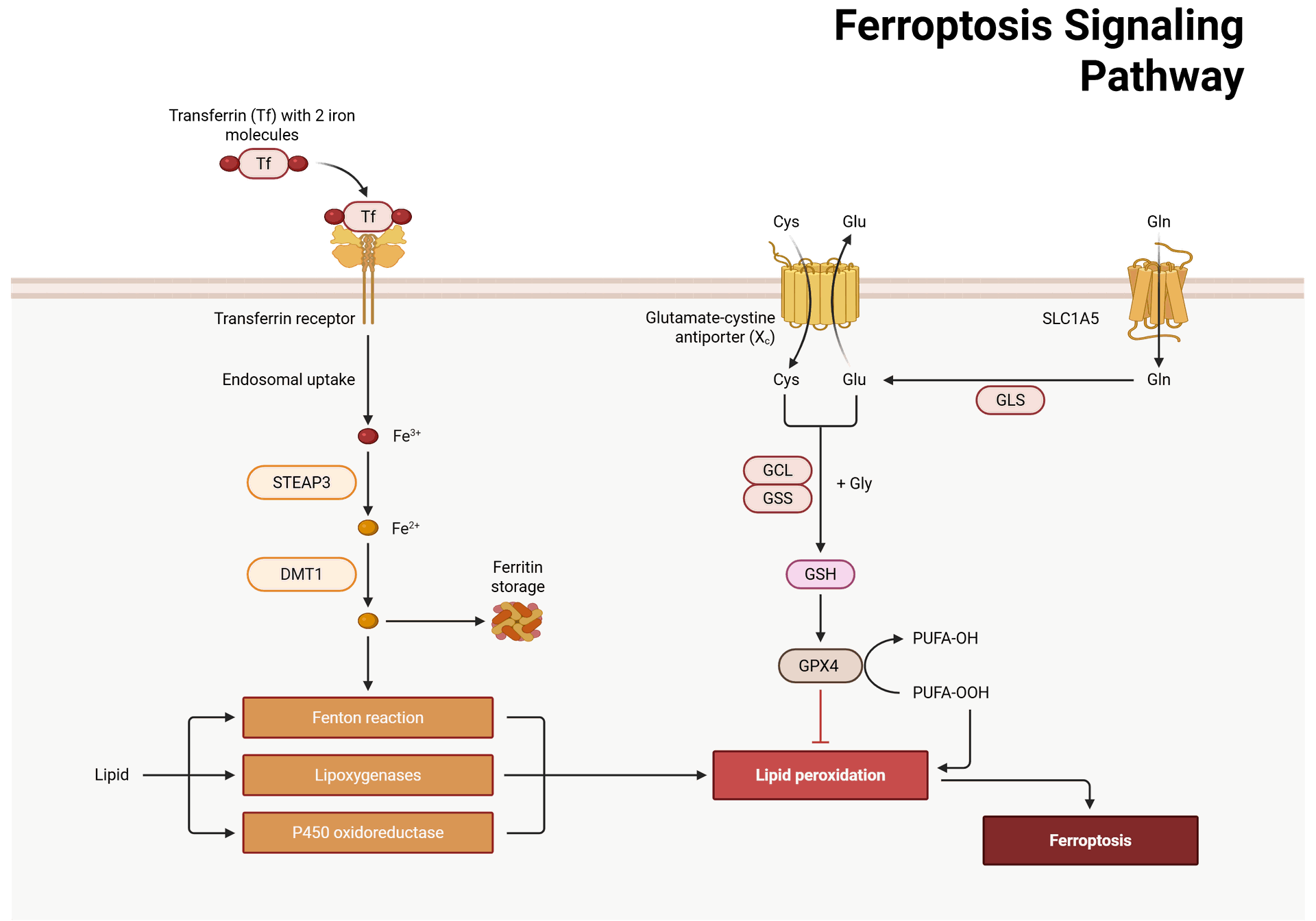
Key Mechanisms Involved in Ferroptosis
Iron Metabolism and Lipid Peroxidation
- At the heart of ferroptosis is the metabolism of iron. Iron plays a pivotal role in the Fenton reaction, where ferrous iron (Fe²⁺) reacts with hydrogen peroxide (H₂O₂) to generate highly reactive hydroxyl radicals (•OH). These radicals can attack lipids in the cell membrane, initiating lipid peroxidation. This peroxidation generates lipid hydroperoxides, which accumulate to lethal levels, causing cell membrane damage and rupture.
- The balance between iron import, storage, and export is crucial for ferroptosis. Excessive iron accumulation within cells promotes oxidative stress, whereas impaired iron export or storage also enhances ferroptosis.
Glutathione Peroxidase 4 (GPX4)
- GPX4 is a critical enzyme that protects cells from ferroptosis by reducing lipid peroxides. GPX4 utilizes glutathione (GSH) as a cofactor to neutralize lipid hydroperoxides. When GPX4 activity is inhibited or when cellular levels of GSH are depleted, lipid peroxidation accelerates, triggering ferroptosis.
- Therefore, GPX4 acts as a central protector against ferroptosis. Its inhibition has been shown to sensitize cells to ferroptotic cell death, which is a strategy that has been explored for cancer therapy, where ferroptosis is used as a way to induce tumor cell death.
System Xc and Glutathione Synthesis
- The System Xc antiporter plays a crucial role in maintaining intracellular levels of cysteine, which is necessary for the synthesis of glutathione (GSH). GSH is a major antioxidant that scavenges reactive oxygen species (ROS) and protects against oxidative damage.
- When System Xc is inhibited, cysteine uptake is reduced, leading to lower GSH levels and consequently increasing the vulnerability of cells to oxidative stress and ferroptosis. This pathway is often targeted in cancer therapy, as some tumors are particularly sensitive to GSH depletion.
Lipid Peroxidation and Ferroptosis-Inducing Pathways
- Lipid peroxidation is the key driver of ferroptosis. Polyunsaturated fatty acids (PUFAs) in cell membranes are particularly prone to oxidation due to their double bonds. In the presence of excessive iron and ROS, these PUFAs undergo lipid peroxidation, generating toxic lipid hydroperoxides that damage cellular membranes and initiate ferroptotic cell death.
- The enzyme ACSLC (Acyl-CoA synthetase long-chain family member 4) has been implicated in enhancing lipid peroxidation, particularly by activating PUFAs. Furthermore, lysophosphatidylcholine (LPC), a byproduct of lipid metabolism, has been found to promote ferroptosis by enhancing lipid peroxidation in the presence of iron.
Ferroptosis Regulators and Signaling Pathways
- Several signaling pathways contribute to the regulation of ferroptosis. For example, p53, a tumor suppressor protein, can activate ferroptosis by upregulating the expression of sestrin 2, which in turn inhibits System Xc and reduces GSH levels. In contrast, Nrf2, a key antioxidant regulator, can protect cells from ferroptosis by upregulating antioxidant defense mechanisms, including the expression of GPX4.
- Additionally, mitochondria play a significant role in ferroptosis regulation. Mitochondrial dysfunction has been linked to the accumulation of ROS and lipid peroxidation, further promoting ferroptosis. Moreover, mitochondrial ferritin, a protein involved in iron storage, can also modulate ferroptosis by controlling intracellular iron levels.
Ferroptosis in Disease
Cancer
In cancer, cells often develop resistance to cell death mechanisms, including apoptosis. Tumors with high iron levels or dysregulated oxidative stress pathways may become resistant to chemotherapy and radiation therapy. However, ferroptosis offers a new avenue for cancer therapy. By inducing ferroptosis in cancer cells, it is possible to bypass resistance mechanisms and promote cell death in a controlled manner. Some chemotherapeutic agents and small molecules have been developed to specifically induce ferroptosis in cancer cells.
Neurodegenerative Diseases
Ferroptosis has been implicated in various neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS). In these conditions, excessive oxidative stress and iron accumulation contribute to neuronal cell death, exacerbating disease progression. By targeting the pathways that regulate ferroptosis, it may be possible to slow the progression of these diseases and offer new therapeutic strategies for neuroprotection.
Liver and Kidney Diseases
Ferroptosis has been implicated in liver injury, particularly in conditions like non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD), where excessive iron accumulation and oxidative stress lead to hepatocyte death. Similarly, in kidney diseases such as acute kidney injury (AKI), ferroptosis can contribute to tubular cell death and renal damage.
Ischemia-Reperfusion Injury
Ischemia-reperfusion injury, which occurs when blood supply is restored to tissues following a period of ischemia (such as during a stroke or heart attack), can lead to ferroptosis due to oxidative stress and iron overload. The restoration of blood flow exacerbates oxidative damage, triggering ferroptosis in endothelial cells, neurons, and cardiomyocytes. Targeting ferroptosis could help mitigate the damage associated with ischemic injuries and improve tissue recovery.